
What Is A Fuel Cell?
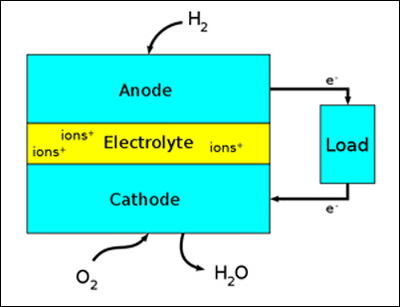
A fuel cell is an electro-chemical device that produces electricity. Being a bit more specific, a hydrogen fuel cell converts hydrogen and oxygen gases into direct current (DC) electricity with ordinary water and heat as byproducts. Another type of electro-chemical device that produces electricity is the battery, think of a flashlight battery. The basic difference in principle is that a battery has all its chemicals stored inside and when the chemicals are all used up, it goes "dead" and is either thrown away or needs to be recharged.
On the other hand, a fuel cell has its fuel, hydrogen and oxygen gases, constantly flowing into it and as long as the flows continue, electricity is produced. At the left is a simplified fuel cell diagram showing the three major components - an anode, a cathode, and an electrolyte. Both the anode and cathode are made out of porous conductive materials so that the gases can flow through them. They are called "gas diffusion electrodes" and are usually a conductive polymer made of carbon composites.
The electrolyte is the "key element" as it is a one way street. Only positive hydrogen ions can pass through the electrolyte to the cathode. The free negative electrons that are formed at the anode must go out through the external circuit to the positive cathode, thus making electricity. External oxygen is also fed into the cathode. When the free electrons reach the cathode, they recombine with the positive hydrogen ions and oxygen to form water vapor (or liquid water) which exists out of the cathode. Fuel cells are usually named after the type of catalyst that is used. While there are many types of fuel cells, for our purposes we will talk primarily about the PEM hydrogen fuel cell and also solid oxygen fuel cells (SOFCs). PEM fuel cells are very close to mass production and are used in stationary, vehicle and other mobile applications. Top
How Exactly Do PEM Fuel Cells Work?
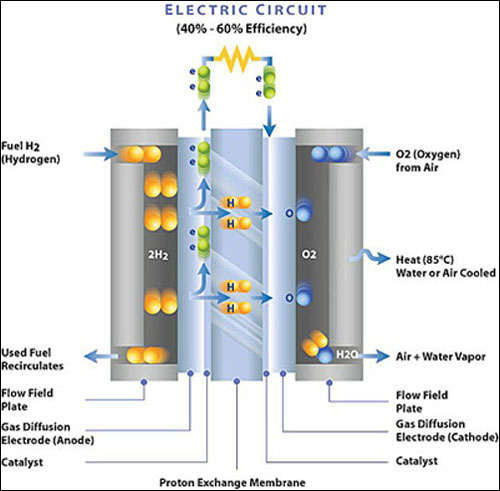
PEM stands for Polymer Exchange Membrane, however originally it was called a Polymer Electrolyte Membrane (note both names have the same acronym). Most electrolytes in fuel cells are liquid. However, a PEM electrolyte is a very thin (just a few sheets of copy paper) solid organic compound with the consistency of a plastic wrap material.
The most common material used for a PEM membrane is Nafion made by DuPont. Nafion is a polymer similar in chemical composition to Teflon. Nafion must be kept moist using liquid water in order to transport the hydrogen protons. This means the PEM has an upper temperature limit of about 90 degrees C because otherwise the membrane would dry out (100 degrees C is the boiling point). Also the PEM membrane must be kept quite thin as thickness increases flow resistance which reduces the flow of the hydrogen protons.
Thanks to Ballard Power Systems (a world leading fuel cell producer located in B.C. Canada) for the fuel cell diagram to the left. Here are the details that make a PEM fuel cell work:
1. Hydrogen gas (H2) under pressure is channeled throughout the anode gas diffusion electrode. The gas diffusion electrodes are also "current collectors" that supply the current to and from the external circuit.
2. Between the anode and the PEM electrolyte is a catalyst which is a very thin coating of platinum powder mounted on very thin carbon paper. The catalyst layer is rough and porous to expose the maximum amount of platinum surface area to the hydrogen. A catalyst is never used up, it just helps the chemical process take place. The platinum catalyst accelerates the separation of the hydrogen gas into two negatively charged free electrons and two positively charged hydrogen proton ions.
3. The positively charged hydrogen protons travel through the moist PEM membrane to the cathode.
4. The free electrons can not pass through the PEM and must exit the fuel cell and travel through the external load to the cathode creating a current (similar to a battery).
5. Oxygen gas (or normal air) is pressurized and channeled throughout the cathode gas diffusion electrode.
6. The oxygen also comes in contact with a second very thin coating of platinum catalyst between the cathode and the PEM. The catalyst accelerates the separation of the oxygen gas into two oxygen molecules. Actually, the hydrogen catalyst side is easier to make work, the oxygen catalyst side is more difficult. Platinum is very, very expensive and is the dominent cost in a fuel cell. See Lab Advances on the Solar In-depth page.
7. Finally all three elements, (hydrogen protons, free returning electrons, and oxygen molecules) combine inside the cathode to form a water molecule (H2O). Water can be either in the form of water vapor or liquid water. Heat is also created by this chemical reaction, which can be used outside of the fuel cell or just exhausted.
8. On the ends of the cell are "flow field plates" usually made of metal. These channel the hydrogen and oxygen into the electrodes as well as allow the water and heat to escape. The flow field plates also give the fuel cell its shape and support strength. Top
PEM Fuel Cell Operation
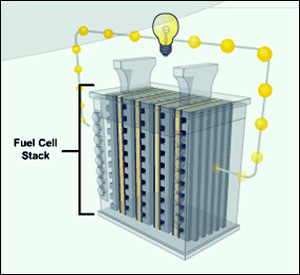
A single fuel cell produces a voltage of 0.6 to 0.8 volts, not enough to power much of anything. So, fuel cells are connected in series to increase the voltage to a reasonable amount. Since power equals voltage times current, cells are also connected in parallel to increase the current for maximum power. The cell current is proportional to the surface area of the cell. The greater the surface area the greater the current. However, physical size is usually a consideration (as in an automobile engine compartment) so compromises have to be made depending on the application. Although at times a bit confusing, the term "fuel cell" is commonly used to designate a single cell as well as a "stacked" set of cells.
One of the attractive characteristics of the PEM fuel cell is that it operates at relatively low temperatures - 50 to 100 degrees C. (Other types of fuel cells operate between 500 and 1,000 degrees C.) A typical fuel cell running at 0.6 to 0.8 volts has an efficiency of about 50% (plus or minus 10%), which means 50% of the hydrogen energy is converted into electricity and 50% into heat. Compare this to the efficiency of a gasoline engine which is about 20%. PEM fuel cell stacks are generally compact and relatively light weight. A fuel cell stack may contain literally hundreds of individual cells. This "scalability" makes fuel cells ideal for a wide range of applications from homes (1-5 Kw), to vehicles (50 to 125 Kw), to electrical generation plants (up to 250 MW or more). Top
Fuel Cell Applications:
Backup/Distributed Power Generation

Backup Power - Several segments of commercial markets are very sensitive to occasional grid blackouts to the extent that they are willing to incur the expense of backup power. Fuel cells are an excellent choice verses batteries. Batteries have a shorter life and need recharging, replacement, and maintenance. Probably the two most sensitive sectors as far as backup is concerned are telecommunications and data centers. Long outages are very disruptive and can have a significant economic effect. Shown at the left is a fuel cell backup center for Verizon in New York City. A blackout in certain critical locations can wreck havoc in a telecommunications network.
Likewise online data centers such as Google, AOL, or the New York Stock Exchange require backup systems. Other commercial industries that require backup are casinos, grocery stores (frozen food areas), railways, mining, ski resorts, hotels, and amusement parks. Also, some foreign countries have unreliable grids - India is one where backup is used extensively. A telecom backup power study estimates that over a 15 year life, a 5 Kw fuel cell backup is 35% less expensive than batteries including US Government incentives of $1,500 per Kw. Without incentives, the savings are 18%. See Telecom Backup Study.

Distributed Power - Another interesting adjacent market is "Distributed Power Generation". This is not backup power, but is power for peak loads or for supplemental power in general. Chemical companies that create hydrogen as a byproduct as part of their main business are the best targets. These companies basically produce free hydrogen which can then be converted into "almost free" electricity. See Ballard Power Systems Distributed Power Example at K2 Pure Solutions in California. In August, 2010 Ballard also delivered the world's largest fuel cell generator, a one MW system, to First Energy Generation Corp. in Ohio. This system is completely portable (see picture on the left) as it is the size of a tractor trailer and it is on wheels. It will be used by First Energy for peak loads on hot days and can be moved to the south during winter. It can supply enough energy to power 500 homes. See World's Largest Hydrogen Fuel Cell. and click on the video. Top
Bloom Boxes (SOFCs)

Bloom Energy Corporation of Sunnyvale, California is a very interesting company. It is a private, venture capital backed company whose major investor is Kleiner Perkins.
Bloom Energy is doing very well with large California customers installing many of their Energy Servers known as "Bloom Boxes" that generate electricity using solid oxide fuel cells (SOFCs). These are on-site generators (distributed power) about the size of a parking space and about 6 feet high that are off the grid.
Solid oxide fuel cells work on the same principles as other fuel cells except the customer can choose one of several available fuels: natural gas, bio gas, propane, hydrogen, or methane. Customers normally choose natural gas as fuel and cut residue carbon dioxide gases in half compared to utility natural gas generators. Each box produces 100 KW of electricity and costs about $1,250,000 (including warranties) before incentives. After US Government and California incentives, the boxes cost about $625,000 not including sales taxes.

The fuel and oxygen do not burn, the boxes produce electricity electro-chemically by converting the chemical energy of the fuel directly into electrical energy with an efficiency of 50 to 55%. Electricity is generated from thin white ceramic plates which are claimed to be made from inexpensive "beach sand". CEO KR Sridhar holds a SOFC ceramic plate at the left. Each ceramic plate is coated with a green ink on one side (the anode) and black ink on the other side (the cathode) so that there are no expensive materials involved.
SOFCs operate at a very high temperature, approximately 800°C, which has been the major obstacle in the past for SOFCs, i.e. to operate at such an extreme temperature in a local business. Bloom Energy claims to have accomplished this and says with incentives they can operate at a cost of electricity that is much less than the going rates in California. Adobe Systems claims it is producing electricity for 8.5 cents per kilowatt versus 13 cents a kilowatt from PG&E and Adobe uses the more expensive bio gas.
Bloom Energy has shipped over 150 Bloom Boxes almost exclusively to California commercial customers. Some of Bloom Energy's customers include Google, Walmart, Ebay, Adobe Systems, FedEx, Bank Of America, Coca-Cola, Caltech, Kaiser Permanente, Staples and other well known companies. Bloom Energy claims that twenty percent of their cost savings comes from avoiding transmission losses on the grid. Bloom Energy also has a program called "Bloom Electrons" where they sell only electricity on a ten year contract (no capital expense involved) and Bloom will manage and maintain the systems. Bloom Energy quadrupled their manufacturing facilities in Sunnyvale and hired about 1,000 people.
Bloom Energy is also looking into military applications. It should be noted that solid oxide fuel cells and PV solar cells are very complementary technologies. They could be used together to provide around the clock power to commercial and utility customers. Bloom Energy has also indicated that at some time in the future they will offer "home" boxes that can safely operate in a residential environment. This is probably five to ten years away. There is speculation that Bloom Energy may go public in 2014 or 2015. Top
Material Handling Fork Lifts

Another very good market for fuel cells is material handling fork lifts. The warehouse market is particularly attractive as PEM fork lifts have quite a few advantages over battery operated fork lifts. Like batteries, PEM cells are quiet and do not emit any noxious gases. But unlike batteries, PEM cells can be rapidly refueled in only two minutes. Whereas battery swapping can take 20 to 30 minutes. Then the batteries must be filled with water and recharged. Batteries last abut 6 hours versus 12 hours for a PEM cell. Downtime is very important in warehouses that operate full shifts and utilize several fork lifts. The voltage delivered by a PEM cell is constant whereas the voltage from a battery decreases as the battery wears down slowing down operations. With no need for battery chargers, changing areas, and battery storage areas, more warehouse space is available.
Finally, because the costs of maintenance and operation are far less for a PEM cell, total 15 year life cycle costs are very competitive with battery operated fork lifts. In addition, it is estimated that fuel cell fork lift systems emit 60% less greenhouse gases than battery systems. Many companies want "visible proof" that they are "green". Fuel cell fork lifts are beginning to be sold at a very brisk pace.
By 2013 there where over 4,000 fuel cell forklifts used in the US. Fuel cell fleets are currently being operated by a number of companies, including Sysco Foods, FedEx, Coca-Cola, Kimberly Clark, and Whole Foods. Wal-Mart plans to purchase over 2,200 fuel cell fork lifts by the end of 2014. As a result most material handling companies are at least studying the use of fuel cell technology for material handling fork lifts.
Fuel Cell Buses

There is considerable interest by several countries in exploring the feasibility of zero emission fuel cell powered buses. One diesel bus generates the equivalent emissions of about 1,000 cars. Cities with serious smog problems all over the world are interested in a fleet of fuel cell buses. Countries like Canada, the UK, Germany, the Netherlands, Brazil, Japan and the State of California have all funded feasibility studies to see if fuel cell buses make sense. Over the years there have been about 300 buses deployed.
Between 2012 and 2015, a total of thirty experimental fuel cell buses (most of which also have electric batteries, making them hybrid buses) are will be tested in a variety of American cities. Buses run on controlled routes and require only one filling station per terminal. Specially trained maintenance crews are readily available. In addition fuel cell buses would be used inside city limits with a top speed of about 40 miles per hour. Some of the issues in different geographic climates are air conditioning, heating, and operation on slippery roads. The main problem with fuel cell buses is higher general maintenance costs and higher purchase prices.
However, the US Department of Energy published a report titled "Fuel Cell Buses in the U.S. Transit Fleet - Current Status 2012" which found that the fuel economy for fuel cell buses to be twice that of diesel. Based upon a one year study of a fuel cell electric vehicle fleet, the report fiound that fuel cell electric buses had a fuel economy about 2.0 times that of conventional diesel buses and natural gas buses. The bus fleet in the study travelled 250,000 miles and logged almost 25,000 hours of fuel cell system operation. Fuel cell bus cost issues remain to be worked out. Top
Fuel Cell Cars
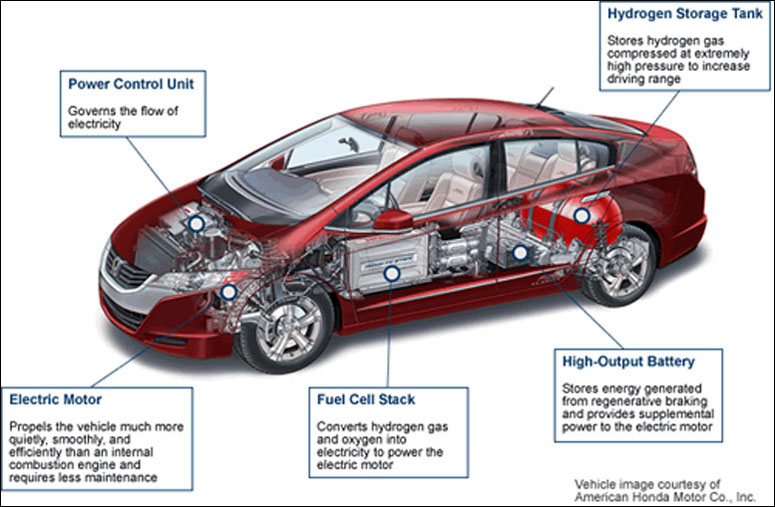
All the major car manufacturers have demonstration fuel cell cars. These include Ford, Chevrolet, Mercedes Benz, Hyundai, Honda and Toyota. There are hundreds of fuel cell cars being test driven. It is generally accepted in the auto industry that in the long run, fuel cell technology will out do battery hybrids and plug in electric cars. While the long term possibilities for fuel cell cars may be promising, there are a number of challenges facing the industry. Product cost is the most significant. The platinum used as a catalyst in a PEM fuel cell is very expensive. Today's fuel cell cars use about 30 grams of platinum per car, auto engineers think that they can get this down to 2 grams in about 10 years. Other fuel cell items are also high cost in low volumes. So until there are significant volumes, cost will continue to be the major impediment to wide spread usage.

Another impediment to wide acceptance of fuel cell cars is hydrogen filling stations. It is estimated that there are about 120 hydrogen filling stations nationwide, most of them in California. However given the size of the United States, many thousands are necessary for this technology to be a success. Countries in Europe and Japan have a much easier time establishing a fuel cell filling station infrastructure and we may see automobile fuel cell success there first.
However, some of the noteworthy advantages of fuel cell cars are that their range is 240 miles per fill up, which is 2 to 3 times that of an all electric car. Also, the life of a fuel cell is much greater than that of a battery. Finally the emissions footprint of fuel cells is much better than that of batteries. This is why, in the long run, most analysts expect fuel cell cars to be the answer to our oil dependent economy and our greenhouse gas problems.
Currently most fuel cell cars are experimental and are available only as "leases". While commercial "sales" may start in 2015 (by Toyota and others), it will be some time before costs are competitive and filling stations are easy to find. Top
Other Fuel Cell Applications
- Scooters - Most scooters are major polluters. A two cylinder scooter produces as much pollution as a heavy duty truck. Scooters are prime targets for fuel cells in India and the rest of Asia where they are very popular.
- Landfills/Waste Water/Breweries/Wineries - All these facilities have one thing in common and that is they all generate methane gas as a by product. Methane gas is rich in hydrogen and thus they are targets for fuel cells.
- Portable Power - Fuel cells can provide power where there is no grid. In addition to remote cabins, campgrounds, and construction power tools, the military has needs for power in battlefields and other situations.
- Consumer Electronics - Fuel cells are expected to radically change small electronic devices. Fuel cells can power a cell phone for 30 days, a laptop for 20 hours, and also provide silent small power tools. These products would run on methanol (basically windshield wiper fluid) rich in hydrogen and be charged from very small charging devices.
- Homes - Fuel cells can be used to provide night time power to houses and businesses that use solar power during daylight hoursExcess electricity from solar panels can be used to separate regular water into hydrogen and oxygen which would be stored in canisters in areas such as basements. At night, these gases can be fed into fuel cells to generate electricity. Several companies are working to develop home fuel cell products.